HOME > LIFE SCIENCE RESEARCH
The Baltic Life Science Map 2023 represents our endeavor to comprehensively analyze the burgeoning healthtech market and innovative enterprises within the Baltic region. This research initiative involves the meticulous aggregation of data pertaining to various projects, technologies, startups, and key stakeholders dedicated to enhancing the quality of life, improving healthcare accessibility, fostering medical tourism, extending longevity, and fostering an innovative ecosystem that supports Life Science startups. The culmination of this research will be a consolidated visualization, the Baltic Life Science Map 2023, which will serve as a singular resource for all ecosystem participants. Our initial research phase is dedicated to the Latvian Life Science ecosystem. |
THE OBJECTIVES OF THIS RESEARCH ENDEAVOR ENCOMPASS:
– Familiarizing stakeholders and professionals within the healthcare, health preservation, and life quality enhancement industries with innovative technologies either under development or already established within Latvia.
– Demonstrating the myriad of solutions aimed at addressing contemporary challenges within the healthcare sector.
– Compiling a comprehensive repository of services and infrastructure available to innovative healthcare enterprises in Latvia, with the aim of fostering future collaborations and joint initiatives.
– Identifying areas of growth and potential national specialization in the global competitive landscape. For instance, we perceive significant potential for the development of medical tourism in Latvia; however, this necessitates concerted efforts, collaboration among diverse stakeholders, and the implementation of cutting-edge technologies within the healthcare system.
RESEARCH METHODOLOGY:
Our research methodology is rooted in a comprehensive analysis of innovative companies within Latvia and a study of global trends in the Life Science market.
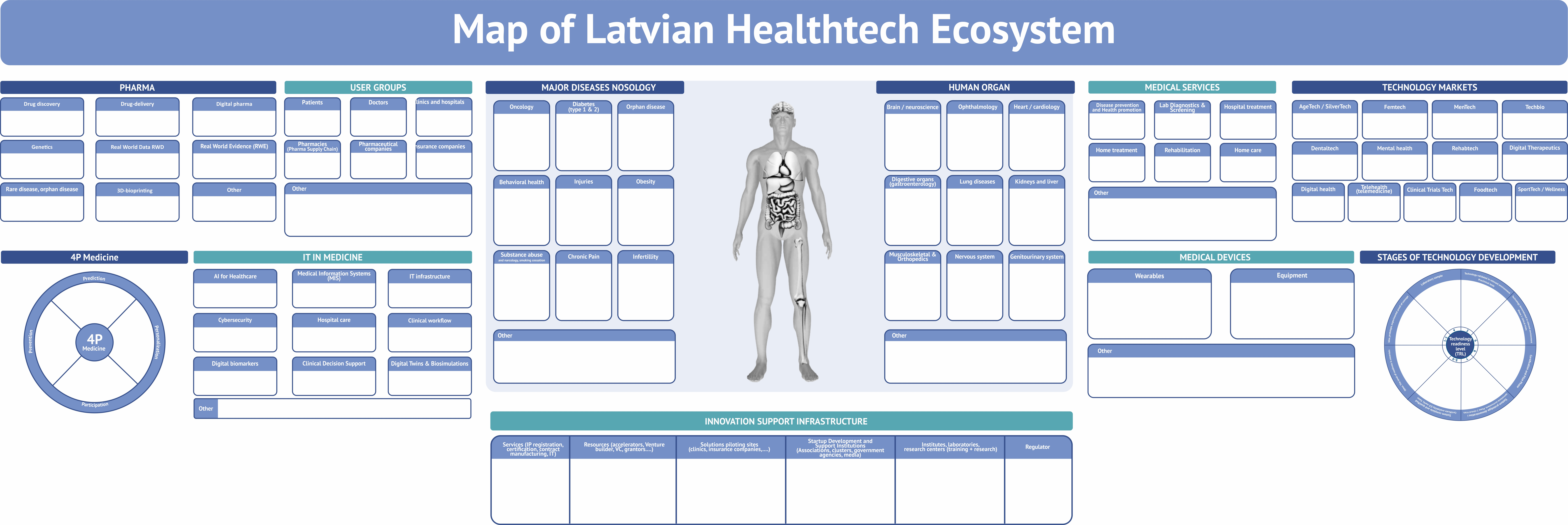
To provide a multifaceted view of the modern healthcare innovation ecosystem, we have categorized technologies into 11 sections:
Technologies and solutions grouped by users:
– patients,
– doctors,
– clinics and hospitals,
– pharmacies (Pharma Supply Chain),
– pharmaceutical companies
– insurance companies,
– others
Description (main technologies involved)
For doctors: Assisted Prescribing, Clinical Decision Support, Computer-Assisted Physician Documentation
For pharmacies (Pharma Supply Chain): Asset Tracking, Autonomous Pharmacy, Blockchain for Shipping, Cold Chain Packaging, Digital Pharmacy, Inventory Management Software, Last-mile Delivery, Medication Adherence, Price Transparency, Robotic Fulfillment, Visibility Platforms
On the processes of providing medical services
Technologies and solutions grouped by the type of service:
– Disease prevention and Health Promotion,
– Lab Diagnostics & Screening,
– Hospital treatment,
– Home treatment,
– Rehabilitation,
– Home care
Description (main technologies involved)
Lab Diagnostics & Screening: Genetic diagnostics, Point-Of-Care Diagnostics
Home care: Full-stack Home Care, Telehealth, Homecare workflow, Dialysis & Infusion Therapy, Care Management Platforms, Medication & DME Fulfillment, Remote Patient Monitoring, Virtual Care, In-home Diagnostics, Home Care Management Platforms, Patient Directed Scheduling & Navigation, Care Coordination & Care Management
Rehabilitation: Post-Stroke & Physical Rehabilitation, Rehab and Mobility-Assist Robotics
This section covers technologies for drug invention, dietary supplements, supporting development processes, digitalization of processes for pharmaceutical companies and pharmacies
– Drug discovery
– Drug-delivery
– Digital pharma
– Genetics
– Real World Data (RWD)
– Real World Evidence (RWE)
– Rare disease, orphan disease
– 3d-bioprinting
– Other
Description (main technologies involved)
Drug discovery: 3D Bioprinting, Organoids& Organ-on-a-chip, AI-Powered Discovery, Single-Cell Analysis, Bio-Simulation, Protein Discovery & Design, Synthetic Biology
Drug-delivery
Digital pharma
Genetics: Gene Editing, Genetic Screening & Testing, Molecular Diagnostics, Genomic Profiling
Real World Data (RWD)
Real World Evidence (RWE)
Rare disease, orphan disease: Rare diseases are those with an incidence of less than one in every 2,000 people in Europe. Orphan diseases are poorly researched conditions, diseases for which specific treatments are not yet known, and diseases of limited interest to scientists and doctors. Patients with such diseases often feel neglected and “lost” in the world of health care.
3d-bioprinting
Other
These markets highlight various analytical resources, such as Sifted, CB Insight and others, to attract the attention of investors.
Description (main technologies involved):
AgeTech/SilverTech – Solutions for people 60+
FemTech and Men’s health – Women’s health technology: Fertility (incl. Screening & diagnostics and Tracking & monitoring), Gynecological health, Menstruation, Menopause, Sexual health & wellness (incl. Contraceptives), Maternal & fetal health. Technology for men’s health
EduMedTech – educational technologies for healthcare
Techbio – The focus is on the ‘technology’ aspect of biotechnology – think of start-ups developing platforms and infrastructure to improve drug discovery, create new food ingredients or reduce our impact on the climate.
Dentaltech – Transforming dental care. Dental prevention and treatment technologies
Mental health – Mental health
Rehabtech – Technology for rehabilitation
Digital health – Digital Health
Digital Therapeutics – Digital therapy
Telehealth (telemedicine) – Health Care Delivery Using Telecommunications Technology: Telemedicine Providers, Platforms & Marketplaces (incl. Hybrid In-Person/Virtual Care), Telepharmacy, Teletherapy, Coaching & Care Management (incl. Direct-to-Consumer), Virtual/Digital Care Enablement, Remote Monitoring, Screening and Diagnostics, Teletherapy, Wellness Coaching and Chronic Care Management, Telehealth Software and Connectivity Solutions
Clinical Trials Tech – Clinical Trials Technology
Foodtech – Healthy Nutrition
SportTech / Wellness – Technology for an Active Lifestyle
– Oncology
– Diabetes (Type 1 & 2)
– Orphan disease
– Behavioral health (incl. Anxiety & Depression, Sleep)
– Injuries
– Obesity
– Substance Abuse and Narcology, Smoking Cessation
– Chronic Pain
– Оther
– Brain disease / Neuroscience
– Ophthalmology
– Heart diseases / Cardiology
– Digestive / Gastroenterology
– Lung diseases
– Kidneys and liver
– Musculoskeletal & Orthopedics
– Nervous system
– Genitourinary system
– Other
Description (main technologies involved)
Brain – Global incidence and prevalence of neurological disorders such as dementia, epilepsy, Parkinson’s disease, Huntington’s disease, Alzheimer’s disease, headaches, migraine, multiple sclerosis, neuroinfections, stroke and cerebral palsy, mental health (Anxiety & depression, Sleep, schizophrenia, etc.)
– Idea, prototype, experimental proof of concept (TRL 1-3)
– Laboratory sample (TRL 4)
– Technology in relevant environment. Preclinical tests (TRL 5)
– Technology demonstrated in relevant environment. Phase 1 clinical trials (TRL 6)
– Certification and Pilot Phase (TRL 6)
– System prototype demonstration in operational environment. Phase 2 clinical trials (TRL 7)
– System complete and qualified. Certificate availability (CE mark). Sales validated (TRL 8-9)
– Other. The level of readiness is uncertain
– Sensors and wearable devices
– Equipment
Description (main technologies involved)
Section includes wearables and equipment for:
– Remote Patient Monitoring
– Point-of-care Testing
– Virtual Reality Diagnostics & Therapeutics
– 3D and Bioprinted Devices
– Post-stroke & Physical Rehabilitation
– Smartphone-based Diagnostics & Therapeutics
– Wearables & Sensors
– Surgical Intelligence
– Computer-aided Diagnostic Imaging
– Glucose Monitoring Technology (Noninvasive Glucose Detection)
– Wireless Connectivity
– Bioelectronic Neuromodulation
– Robotic and Image-guided Surgery
– Rehab and Mobility-Assist Robotics
– AI/ML for Healthcare
– AR/VR
– Medical Information Systems (MIS)
– IT infrastructure
– Cybersecurity
– Hospital care
– Clinical workflow
– Digital biomarkers
– Clinical Decision Support
– Digital Twins & Biosimulations
– Other
Description (main technologies involved)
AI/ML for Healthcare
– Admin & Operations (incl. Digital Front Door, RPA, Underwriting & Claims, Revenue Cycle Management, Patient Engagement (Payers), EHR Management and Virtual Scribes)
– Monitoring & Diagnostics (incl. Radiology AI, Pathology AI, Remote Patient Monitoring, Point-of-Care (in clinic))
– Tech Infrastructure (incl. DataOps & Data Science Platforms)
– CareDelivery (incl. Specialty Care, Surgical Tech, Chronic Disease Management, ICU Tech, Primary Care)
– Drug Development & Research (incl. Drug R&D, Medical Data Repositories, Clinical Trial Tech)
– Medical Imaging AI
IT infrastructure
– Quantum Computing
– Cloud R&D Software
Cybersecurity
– Secure Clinical Communication
– Endpoint Security
– Threat Intelligence
– Managed Security
– Third-Party Risk
– Network Security
– SIEM
– Secure Collaboration
– Data Security
– Website Security
– Vulnerability Management
– Cloud Security
– Human Element
– Data Privacy (HIPAA)
– Medical Device Security
Hospital care
– Data&information management
– Diagnostics (Pathology & Radiology)
– Patient intake & virtual waiting rooms
– Referral management
– Revenue cycle management
– Scheduling
– Self-trage & navigation
– Surgical intelligence
– Patient flow
– Patient monitoring
– Patient Data & Analytics
Clinical workflow
– Electronic Health Record Augmentation
– Patient Flow & Command Center
– Provider Staffing & Human Capital Management
– Referral Management
– Pre-Visit Patient Management
– Virtual Assistants
– Provider Search & Scheduling
– Patient Engagement
– Population Health Analytics
– Risk Adjustment Technology
– Utilization Management
– VBC Contract Management
– Clinical Exam Tools
– Self-Triage & Navigation
– Cost Reduction (incl. Care Management & Coordination, Managed Care, Utilization Management, Value-Driven Providers, Remote Patient Monitoring)
– Administartive Tools (incl. Populations Health Analytics, CQM Capture, SDOH Screening & Refferal Management, Contract Management, Risk Adjustment Coding Capture)
Digital biomarkers
– Vital signs
– Sleep
– Mobility and activity
– Visual
– Auditory
– Data analytics & clinical trials platforms
– Sweat & saliva
– Urine & feces
– Blood testing (incl. Blood glucose)
– Innovation recipient/implementator (companies interested in collaboration with startups)
– Services (IP registration, certification, contract manufacturing, IT)
– Resources (accelerators, Venture builder, VC, grantors….)
– Solutions piloting sites (clinics, insurance companies,….)
– Startup Development and Support Institutions
– (Associations, clusters, government agencies, media)
– Institutes, laboratories, research centers (training + research)
– Regulator
– Other
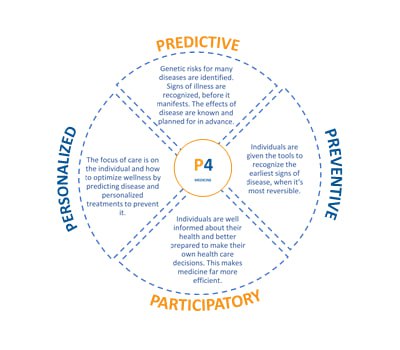
As the healthcare ecosystem continues to evolve, our map will remain dynamic. We are committed to refining the map’s structure and providing greater detail, particularly when multiple companies are engaged in the same domain or market. If you find that a specific category is lacking or if you wish to propose a new section, please reach out to us at info@expanse.vc, and we will certainly consider your input.
OUR CRITERIA FOR INCLUDING A COMPANY/PROJECT ON THE MAP ENTAIL:
– Companies primarily focused on healthcare technology development, serving healthcare stakeholders (suppliers, patients, regulators), or life sciences (pharmaceuticals, medical devices), as well as those offering services or seeking collaboration with development-focused companies;
– A physical presence in Latvia (mandatory for startup companies), either through a headquarters, representative office, or branch office.
– Provision of relevant information about the entity, including a website, contact person, contact details, a brief self-description, and a logo.
– A team comprising a minimum of two members.
If you believe your company merits inclusion on this map or if you wish to update the displayed information, please complete the following form
We extend our gratitude to the Digital Health Cluster, whose invaluable support facilitated the development and data collection for this map:
– Digital Health Cluster https://www.digitalaveseliba.lv/
We envision that the Baltic Life Science Map 2023 will offer a comprehensive overview of startups and the scope of medical technology development. This representation underscores the readiness and potential of the Latvian and Baltic ecosystems, making them appealing destinations for innovative enterprises from around the world.
We sincerely appreciate your collaboration with us in this endeavor!

